Sinovac gets world's 1st production license for A/H1N1 vaccine
China approved Thursday the A/H1N1 flu vaccine produced by domestic pharmaceutical company Sinovac, making it the first to get a production license in the global race.
The State Food and Drug Administration (SFDA) issued the license for Sinovac's vaccine called Panflu.1 on Thursday, after it passed SFDA's experts evaluation on Aug. 31.
"Clinical trials show that Panflu.1 is very safe and its immunogenicity factors reach international standards," said Zhang Wei, director of SFDA's registration department.
Panflu.1 could safely be given to people aged from three to 60 years old in a single shot, 15 microgram dose, according to the evaluation report.
|
|
|
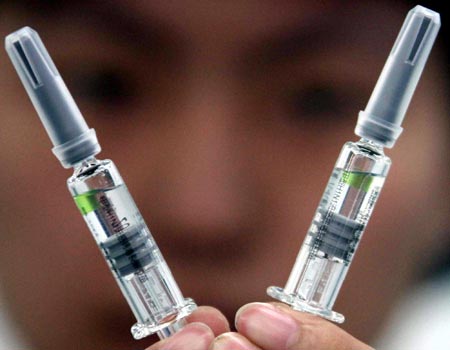
Two A/H1N1 flu vaccines are showed by personnel of Sinovac in Beijing, capital of China, Sep. 3, 2009. [Xinhua] |
Sinovac had the capacity to produce five million doses of vaccine before Oct.1, and 20 to 30 million doses per year, according to its president Yin Weidong.
"Currently Sinovac will focus on meeting the need of the Chinese government. And if we still have extra capacity, Sinovac is quite open to discuss the possible opportunity on A/H1N1 vaccine export," said Yin.
On Wednesday, Sinovac and Boryung Pharmaceutical Company Ltd., a Republic of Korea pharmaceutical manufacturer, reached an agreement to collaborate on possible vaccine supply to the country.
The vaccine would be reserved by the State instead of going on general sale across the country, according to Zhang.
"The government will make out an overall plan and decide certain high-risk groups who will get priority vaccination," Zhang said.
Yin declined to comment on this because "it's inappropriate for us to talk about it as Sinovac is only responsible for production."
Many countries are racing to develop the vaccine against the A/H1N1 flu as experts and officials worldwide fear there will be a flu pandemic in the northern hemisphere during the coming autumn and winter.
Another A/H1N1 flu vaccine, produced by Chinese pharmaceutical company Hualan Biological Engineering Inc.,is also seeking approval this week.
Hualan's vaccine, suitable for all those aged over three years, is waiting for the SFDA's final decision after it passed experts evaluation on Tuesday.
A further nine Chinese pharmaceutical companies are also applying for registration of A/H1N1 flu vaccines. The SFDA is expected to finish all evaluation by mid September, according to Zhang.
"SFDA will tighten the supervision on the vaccine's production and monitor its adverse reactions after large-scale vaccination," Zhang said.
Health Minister Chen Zhu said in August that China would be able to produce enough A/H1N1 flu vaccine for 65 million people by the end of the year.
A World Health Organization (WHO) official was earlier reported to have said the WHO hoped China would donate flu vaccines to help poorer countries.
Nearly 4,000 Infected
As of 3 p.m. Wednesday, China had reported 3,981 cases of A/H1N1 flu on the mainland, of whom nearly 3,400 had recovered, with no confirmed deaths, according to the Health Ministry.
Recent cases of group infection have also highlighted the rising risk of an A/H1N1 flu pandemic in the country.
|
|
|
A/H1N1 flu vaccines are made in Sinovac in Beijing, capital of China, Sep. 3, 2009. [Xinhua] |
On Sunday, a senior high school in central China's Henan Province reported 80 confirmed flu cases, and reported 29 new confirmed cases Tuesday. while a junior high school in northwestern Gansu Province reported 26 cases.
Globally, the disease had killed about 2,000 people and infected more than 180,000. It is circulating in more than 170 countries.
(Xinhua News Agency September 3, 2009)
 0 Comments
0 Comments